Abstract
Introduction
Venetoclax (V), an orally administered, highly selective, potent BCL-2 inhibitor, induces high ORR when given as monotherapy to pts with relapsed/refractory (R/R) CLL, including high-risk populations, e.g. del(17p). V is also well tolerated when combined with rituximab (R) and achieves improved CR rates and minimal residual disease negativity (MRD-). Here, we provide first released data from the primary analysis of MURANO (NCT02005471), the first Phase 3 study of V in pts with R/R CLL, which assessed efficacy/safety of VR vs standard chemoimmunotherapy, bendamustine (B) + rituximab (BR).
Methods
Eligibility for this open-label, randomized, Phase 3 study included R/R CLL requiring treatment (iwCLL guidelines), 1-3 prior lines of therapy (including ≥1 chemo-containing) and ECOG PS ≤1. Prior B was allowed provided response duration was ≥24 mo. Pts were randomized (1:1) to VR or BR. Stratification factors were del(17p), responsiveness to prior therapy and geographic region.
In the VR arm, a 4- or 5-wk graduated dose ramp-up of V from 20-400 mg daily was used to mitigate potential tumor lysis syndrome (TLS) risk. Beginning at Wk 6, R was then given monthly for six 28-day cycles (IV 375 mg/m2 first dose, then 500 mg/m2) in combination with V daily. Pts continued with V 400 mg for a maximum of 2 yr or until disease progression (whichever first). In the BR arm, pts were given B (IV 70 mg/m2) on Days 1 and 2 of each of six 28-day cycles in combination with R using same R dosing schedule. MRD was centrally assessed by ASO-PCR and/or flow cytometry in peripheral blood at screening, Mo. 4 and 9, and 3-monthly follow-up visits.
The primary endpoint was investigator (INV)-assessed PFS. An interim analysis was preplanned at ~140 INV-assessed PFS events. On data review, an Independent Data Monitoring Committee recommended study arms to be unblinded to the sponsor as pre-specified statistical boundaries for early stopping were crossed for PFS in favor of VR.
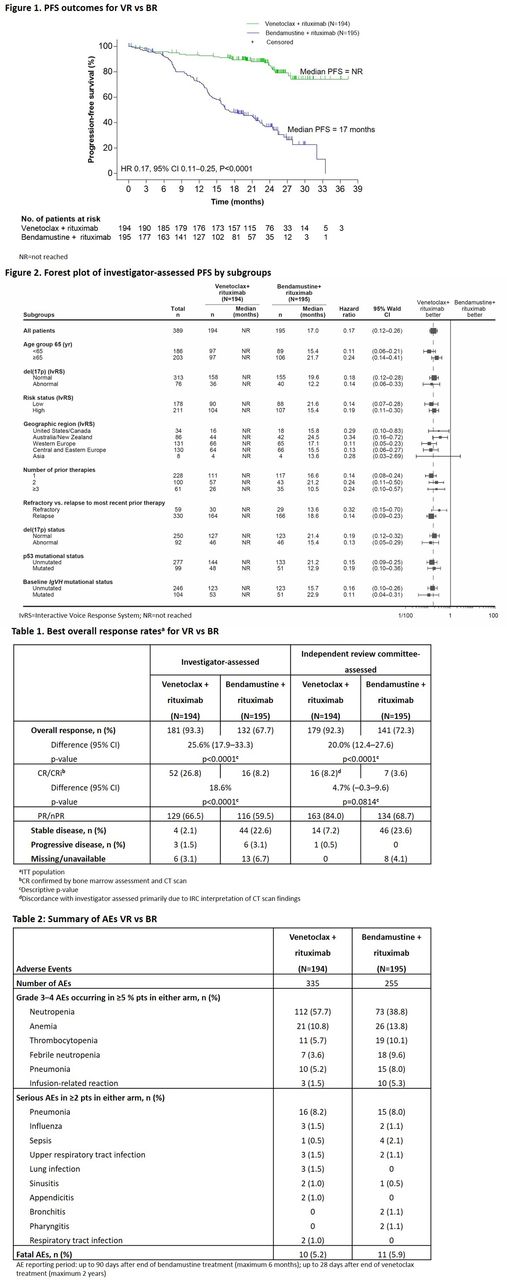
Results
389 pts were enrolled in VR (n=194) and BR (n=195) arms, which were well balanced: median (range) age, 64.5 (28-83) vs 66.0 (22-85) yr; 1 prior therapy, 57.2% vs 60.0%; fludarabine refractory, 14.1% vs 15.5%; del(17p), 26.6% vs 27.2%. At data cut-off (8 May 2017; median follow-up, 23.8 mo. [range 0.0-37.4]), INV-assessed PFS was superior for VR vs BR with HR 0.17, 95% CI 0.11-0.25, P<0.0001; median not reached vs 17.0 mo. (Fig 1). 24-mo. PFS estimates were 84.9% vs 36.3%, respectively. Consistent treatment effects on PFS were observed in all subgroups assessed (Fig 2). With HR 0.19, 95% CI 0.13-0.28, P<0.0001, Independent Review Committee-assessed PFS showed a similar magnitude of benefit. Key secondary efficacy endpoints showed consistent improvements for VR vs BR including a notable improvement in OS (HR 0.48, 95% CI 0.25-0.90). INV-assessed ORR was 93.3% with VR vs 67.7% with BR (Δ=25.6%, 95% CI 17.9-33.3%); CR/CRi was achieved in 26.8% vs 8.2% of pts, respectively (Table 1). Higher peripheral blood MRD- rates attained at any time were seen with VR vs BR (83.5% vs 23.1%; Δ=60.4%, 95% CI 52.3-68.6%) by ITT analysis. MRD negativity was more durable in the VR arm.
Consistent with known safety profiles of the regimens, Grade 3-4 neutropenia was higher in VR arm but there was no increase in febrile neutropenia or Grade 3-4 infection (Table 2). There were 6 (3.1 %) and 2 (1.1%) Grade ≥3 AEs of TLS reported for VR and BR, respectively; one clinical TLS event in each arm (a Grade 4 acute renal failure in BR, transient increase in creatinine in VR; VR event occurred on an earlier 4-week ramp-up schedule). Richter transformation was confirmed in 6 pts and 5 pts for VR vs BR, respectively. AEs leading to death were seen in 5.2% vs 5.9% of pts. Median relative V dose intensity was 97% of protocol-specified drug exposure.
Conclusion
The primary analysis of MURANO, the first Phase 3 study of V in R/R CLL, shows a profound improvement in PFS vs standard BR chemoimmunotherapy, with consistent effects in all-risk subsets. Key secondary endpoints, including OS, ORR and CR rate, also showed consistent improvements with remarkable rates of peripheral blood MRD- that exceed those previously attained in treatment of R/R CLL. This enhanced disease control was achieved in a multinational setting with an acceptable safety profile, without significant TLS, demonstrating that treatment with VR resulted in outcomes superior to that of BR for pts with R/R CLL.
Seymour:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Morphosys: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sunesis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kipps:Celgene: Consultancy; Oncternal: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Eichhorst:Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Hillmen:Celgene: Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria. D'Rozario:Roche: Consultancy. Assouline:Lundbeck: Other: Advisory Board; Paladin: Speakers Bureau; Pfizer: Speakers Bureau; Bristol Myer Squibb: Speakers Bureau; Roche Canada: Consultancy. Owen:AstraZeneca: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Lundbeck: Honoraria; Roche: Honoraria; Merck: Honoraria. Gerecitano:Merck: Consultancy; Mass Medical International: Consultancy; Incyte: Consultancy; Arcus Medica: Consultancy; Aratana: Consultancy; Bayer: Consultancy; Genentech: Consultancy; Samus Therapeutics: Consultancy; Abbvie: Consultancy; Gilead: Consultancy; Orexo: Consultancy. Robak:AbbVie: Honoraria, Research Funding; Akari Therapeutics Plc: Honoraria, Research Funding; Roche: Honoraria, Research Funding. De la Serna:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jaeger:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria; Amgen: Honoraria; AOP Orphan: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; GSK: Honoraria; Infinity: Honoraria; Millennium: Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; Bioverativ: Honoraria, Research Funding. Cartron:Gilead: Honoraria; Janssen: Honoraria; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Sanofi: Honoraria. Montillo:Novartis: Honoraria; Roche: Research Funding; Abbvie: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Humerickhouse:AbbVie: Employment, Equity Ownership. Punnoose:Genentech: Employment. Li:Genentech: Employment. Boyer:Roche: Employment. Humphrey:F-Hoffmann-La Roche: Employment, Equity Ownership. Mobasher:Roche: Equity Ownership; Genentech: Employment. Kater:Roche: Consultancy; Acerta/Astra Zeneca: Consultancy, Research Funding; Roche/Genentech: Research Funding; Abbvie: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Research Funding; Sandoz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal